Bimatoprost
Dermatological indications
Bimatoprost is a prostaglandin analogue
Researchers noticed the drug had the side effect of stimulating eyelash growth and darkening of the eyelashes in patients with glaucoma.
Effect of bimatoprost solution on eyelashes

Basic Info.
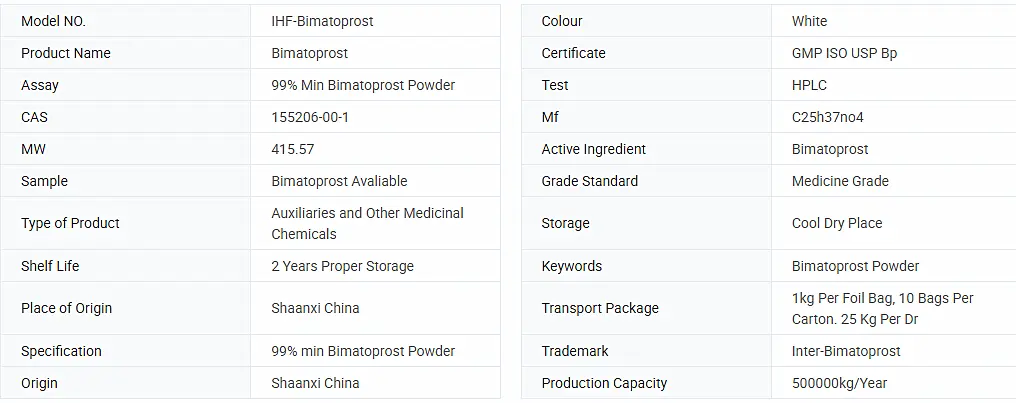
Wholesales Pharmaceutical Cosmetic Raw Material 99% Purity Bimatoprost Powder CAS 155206-00-1 Bimatoprost
| Product name | Wholesales Pharmaceutical Cosmetic Raw Material 99% Purity Bimatoprost Powder CAS 155206-00-1 Bimatoprost |
| Appearance | White powder |
| Molecular formula | C25h37no4 |
| Keywords | Powder Bimatoprost;Material Bimatoprost;Raw Powder Bimatoprost |
| Shelf Life | 24 months when properly stored |
| Storage | Keep in a cool, dry, dark location |
Off-label uses
Bimatoprost has been reported to be effective for eyebrow thinning.
Bimatoprost also has the potential to stimulate the growth of scalp hair. currently studying the safety and efficacy of a new formulation of bimatoprost for use specifically as a topical hair loss treatment for common baldness (androgenetic alopecia) in both men and women.
A pilot study has shown that topical bimatoprost ophthalmic solution 0.03% shows promise as a treatment for localised, stable vitiligo.
How does bimatoprost work?
Bimatoprost is a compound derived from fatty acids designed to bind to prostaglandin receptors.
Prostaglandin receptors are present in the hair follicle.
Bimatoprost is believed to affect the growth of hair follicles by increasing the per cent of hairs in the anagen (growth) phase of the hair cycle and increasing the duration of this phase.
Bimatoprost solution also increases the size of the dermal papilla (hair bulb).
These actions have the effect of making hair longer and thicker.
Bimatoprost also stimulates pigment cells in the skin and hair follicles (melanogenesis) accounting for its observed efficacy in vitiligo.
What precautions should you take when using bimatoprost?
Bimatoprost solution is intended for use on the skin of the upper eyelid margins at the base of the eyelashes.
It should not be applied to the lower eyelid. The bottom lashes receive the drug from the top lashes through blinking.
It is possible for hair growth to occur in areas of skin that bimatoprost frequently touches. Any excess solution outside the upper eyelid margin should be blotted with a tissue or other absorbent material to reduce the chance of this happening.
The applicator should be disposed of after one use. A new sterile applicator should be used for the opposite eyelid margin.
The onset of effect is gradual and is not apparent in the majority of patients until 2 months.
The growth of eyelashes can be expected to gradually return to the original level upon discontinuation of treatment with bimatoprost.
Bimatoprost solution contains benzalkonium chloride, which may be absorbed by soft contact lenses. Contact lenses should be removed prior to application of bimatoprost and may be reinserted 15 minutes following its administration.
Bimatoprost solution should be used under the close supervision of a physician if there is a history of abnormal intraocular pressure (IOP) or other prostaglandin products are being used for the treatment of IOP.
Bimatoprost solution should be used with caution in patients with active intraocular inflammation (e.g., uveitis) because the inflammation may be exacerbated.
Side effects
Clinical study experience
The most frequently reported adverse events (< 4% patients) with bimatoprost solution are:
Itchy or irritated eyes
Red eyes
Darkened skin
Dry eye symptoms
Red eyelids.
Postmarketing experience
The following reactions have been identified during postmarketing use of bimatoprost solution in clinical practice:
Burning sensation (eyelid)
Red eyelids
Eye swelling
Eyelid irritation/itch
Eyelid swelling
Iris darkening
Increased tears
Rash on eyelids and around eyes
Skin discolouration around eyes
Blurred vision.

Use in specific populations
Pregnancy
There are no adequate and well-controlled studies of the use of bimatoprost ophthalmic solution 0.03% in pregnant women. Because animal reproductive studies are not always predictive of human response, bimatoprost solution should be administered during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Lactation
It is not known whether bimatoprost solution is excreted in human milk. Caution should be exercised when bimatoprost is administered to a nursing woman.
Paediatric use
Safety and effectiveness in children have not been established.
Geriatric use
No overall differences in safety or effectiveness have been observed between elderly and other adult patients.
Hepatic impairment
In patients with a history of liver disease or elevated liver enzymes, bimatoprost solution 0.03% had no adverse effect on liver function over a period of 48 months.
Product Package
Storage: Store in a cool, dry, clean place away from moisture and direct sunlight.
Packing: 25kg/drum or according to demand.
Lead Time: 3-7 days.
Shelf Life: 2 years.
Remark: Customized specifications also can be achieved.
.webp)
Contact Us
With the concept of “Quality First, Integrity First”, Hancuikang Bio-Technology Co., Ltd. is strictly performing the standard in production and quality control of Bimatoprost Prostaglandin Receptor Agonist. Independent laboratory and famous third-party retest confirm that each batch is qualified, so please do not doubt, You can contact us by e-mail: fxu45118@gmail.com or Whatsapp/Wechat:86-13379475662









